‘Winkin’, Blinkin’ and Nod’ Nets Major NIH Research Funding
Multimillion-dollar study will reverse-engineer the facial movements mammals use to survive, resulting in a comprehensive 3D brain circuitry atlas
Nov. 2, 2018 | By Cynthia Dillon
Eugene Field’s classic children’s poem "Wynken, Blynken, and Nod" tells the tale of three child-sailors fishing from a wooden-shoe boat under the stars. In the bedtime poem, the young sailors represent the sleepy facial and head movements of children on the verge of dreamland—blinking eyes and nodding heads. Similar face and head movements noticed in mammals as they breathe and rummage for food is the subject of a $15-million study supported by the National Institutes of Health. Scientists from the University of California San Diego, Ben Gurion University, Duke University and Laval University, with support from colleagues at Cold Spring Harbor Laboratories and the HHMI Janelia Research Center, will conduct the research to explore how mammals sniff, nod, and move their faces and mouths—backwards.
Technically, the scientists, led by UC San Diego’s David Kleinfeld, distinguished professor of physics and of neurobiology and Yoav Freund, professor of computer science and engineering, will reverse-engineer the orofacial circuits that govern mammalian behaviors, like essential breathing and searching for food, as part of the Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, an exciting trans-agency effort to arm researchers with revolutionary tools to fundamentally understand the neural circuits that underlie the healthy and diseased brain. The research team will do this by observing the whiskers of rats and mice.
This system of specialized mammalian hairs, called vibrissa, is used for tactile sensing. The vestiges of vibrissae in humans is found above the upper lip—our fingers do the job now. The abilities of vibrissae literally stem from the brain, where each motor action reflects the participation of motor nuclei in cranial circuits, within closed loops, between sensors and muscle actuators. Critically, these loops are also nested and connected to feedback and feedforward pathways that underlie coordination between orofacial motor actions.
To determine how the different actions are coordinated to form a rich repertoire of behaviors like the orchestrated displacements of the head, nose, tongue and vibrissa during activities like food exploration or rhythmic breathing, the scientists will explore the brainstem circuits that drive vibrissae set-point and rhythmic whisking—the repetitive, rapid, back-and-forth sweeping of whiskers mammals display as they move and explore.
The team will work over five years to measure and computationally model the brainstem circuitry and dynamics involved in these essential mammalian movements. Applying technologies such as vivo electrophysiology and optogenetics, the scientists will establish the standard brainstem nuclei connectivity for all related research projects going forward, while their technical methodologies will result in the creation of orofacial circuit wiring diagrams and a 3D atlas that will provide general lessons about the nature of neuronal computation.
“The analytic and anatomical tools developed for these studies ... will be made available through our data science core to the larger neuroscience community,” said Kleinfeld.
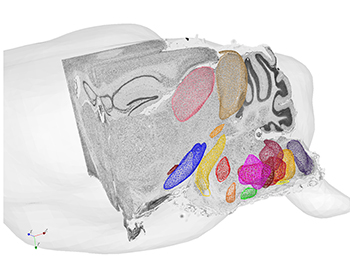
The digital atlas will utilize machine learning to automate anatomical annotation of brainstem nuclei. The atlas also will allow accurate 3D alignment of labeled neurons, while further digitization of serially sectioned brain data sets will afford 3D reconstruction and alignment of small brainstem subregions. Overall, it will enable the collation of this data from different brains into the same atlas.
“The studies will make use of molecular, genetic and functional labeling methods to enable cell phenotyping and circuit tracing, and the sum of these techniques will help elucidate the functions of intrinsic brainstem circuits and their modulation,” explained Freund.
This research is supported by the NIH (grant no. U19 NS107466) with UC San Diego as the lead institute. The content of this news release is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
At UC San Diego, our research efforts are designed to change the world for the better—through new medicines, innovative technologies and more that will help address disease, global security, public policy, climate change and more.
