Researchers Discover How Strep A Bacteria Evade Our Natural Defense Mechanisms
September 21, 2022 | By Michelle Franklin
The world is teeming with bacteria, some helpful and some harmful. Streptococcus pyogenes falls into the latter category. According to the National Institutes of Health, there are over 700 million annual cases of illness attributable to S. pyogenes globally. Although most are not severe, this bacterium is responsible for serious infections including toxic shock syndrome and necrotizing fasciitis, otherwise known as “flesh-eating disease.”
Our bodies have a natural defense against S. pyogenes — a peptide called LL-37. Yet with over 700 million cases annually, S. pyogenes has clearly learned how to disarm LL-37. The mechanism the bacterium uses to do this has never been well understood, but researchers at UC San Diego recently published a paper in eLife shedding new light on this global health threat.
The work was led by Department of Chemistry and Biochemistry Professor Partho Ghosh’s lab, alongside School of Medicine’s Department of Pediatrics Professor Victor Nizet. Together they used X-ray crystallography to examine how the M protein of S. pyogenes disables LL-37.
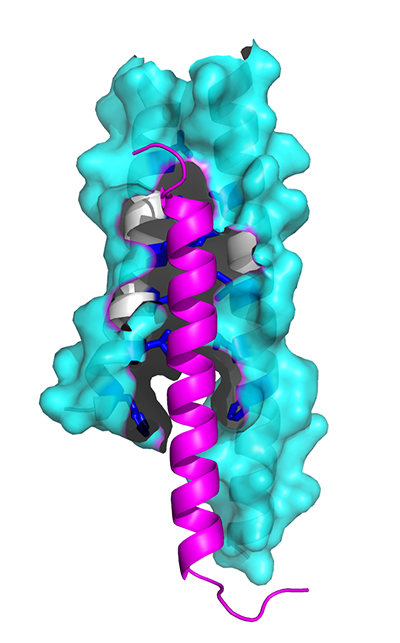
The M87 protein (blue) unfurls to reveal an interior cavity that grabs LL-37 (pink).
(cr: Ghosh lab / UC San Diego).
LL-37 is produced on the skin and kills bacteria by puncturing their cell membrane — similar to a needle popping a balloon — but some strep bacteria are able to capture LL-37 and prevent it from working. Scientists have known that the M protein of S. pyogenes is responsible for capturing LL-37, but it was not known how this was accomplished.
There are over 200 strains of S. pyogenes, each with their own M protein variant. The vast number of different strains is one reason why it is hard to acquire any immunity against group A strep, and one person can be infected over and over again.
Piotr Kolesinski and Kuei-Chen Wang, both postdoctoral researchers in Ghosh’s lab, worked to identify an M protein that would bind to LL-37 and could be crystallized, leading them to single out a particular M protein called M87.
They used X-ray crystallography to visualize, at an atomic level, how the M87 protein is able to capture LL-37. What they learned was that M87 opens up to expose parts that are usually hidden to capture LL-37 — the way a fist unfurls into an open hand — before closing up again. Acting as a sentry, M87 protects the bacterial cell membrane from attack.
The team made a mutation in the protein so that it could no longer capture LL-37. They tested the mutated protein in a test tube, and once they achieved the desired result, worked with Nizet’s lab to test it in the S. pyogenes bacterium itself. The result was promising: with the mutation, the M87 protein was no longer able to stop LL-37 from puncturing the bacterial cell membrane.
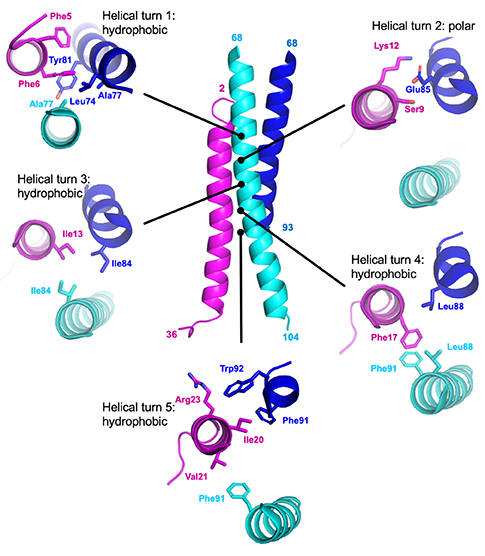
Five points of LL-37 (pink) captured by the M87 protein (light and dark blue) (cr: Ghosh lab / UC San Diego).
Ghosh’s lab studies interactions between bacterial pathogens and humans as a way to understand how these pathogens function and how they can be stopped.
“Seeing is believing. This study has allowed us to see a more precise level of detail about how M87 and other M proteins are able to capture LL-87,” stated Ghosh. “We hope this research will be useful going forward in designing drug therapies that combat the growing number of strep A infections worldwide.”
Ghosh’s lab hopes to help with future vaccine design. Although there are antibiotics that treat strep A infections, there are no vaccines to prevent infections. One reason is that the number of M protein strains make it difficult to create a vaccine effective against all variants. However, during the course of their research, Ghosh’s lab observed 14 other M protein variants of S. pyogenes that used the same binding mechanism as M87 to disable LL-37. This means it may be possible to create a vaccine that could be effective against multiple strains of group A strep, moving the world one step closer to treating a pervasive disease.
Full list of authors: Piotr Kolesinski and Kuei-Chen Wang (Department of Chemistry and Biochemistry); Yujiro Hirose and Victor Nizet (Department of Pediatrics); and Partho Ghosh (Department of Chemistry and Biochemistry).
Funding provided by the National Institutes of Health (R21AI144901).
